Trademark Trial and Appeal Board
Patent and Trademark Office (P.T.O.)
*1 IN RE LINCOLN DIAGNOSTICS, INC.
Serial No. 74/100,207
February 8, 1994
Hearing: August 19, 1993
Angela Micheli
Trademark Examining Attorney
Law Office 11
(Thomas G. Howell, Managing Attorney)
Before Rice, Seeherman, and Hohein
Administrative Trademark Judges
Opinion by Rice
Administrative Trademark Judge
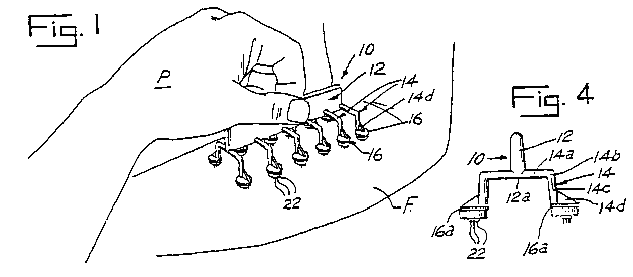
An application has been filed by Lincoln Diagnostics, Inc. to register, on the Principal Register under the provisions of Section 2(f) of the Trademark Act of 1946, 15 U.S.C. 1052(f), the product configuration shown below
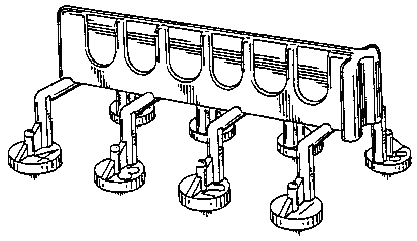
for "medical apparatus used to effect simultaneous, multiple skin tests for detecting immediate hypersensitivity to allergens." [FN1] It is stated in the application that the mark consists of a configuration of a medical apparatus which effects simultaneous, multiple skin tests for detecting immediate hypersensitivity to allergens; that the lining in the drawing is for shading purposes only; and that applicant claims the entire design as its mark.
Registration has been finally refused, under Sections 1, 2, and 45 of the Act, 15 U.S.C. 1051, 1052, and 1127, on the alternative grounds that the configuration sought to be registered is de jure functional, and that even if the configuration is only de facto, rather than de jure, functional, it has not acquired distinctiveness as an indication of origin for applicant's goods.
We turn first to the refusal to register on the ground that applicant's configuration is de jure functional. A configuration which is so utilitarian as to constitute a superior design for its purpose, so that competitors need to copy it in order to compete effectively, is de jure functional (functional in law), and unregistrable. The mere fact that a product configuration has utility does not necessarily mean that the configuration is unregistrable; registrability depends upon the degree of its design utility. See: In re Morton-Norwich Products, Inc., 671 F.2d 1332, 213 USPQ 9 (CCPA1982). Factors which are relevant to the determination of whether a particular product design is superior include (1) the existence of a utility patent that discloses the utilitarian advantages of the design, (2) advertising materials in which the originator of the design touts the design's utilitarian advantages, (3) the availability to competitors of alternative designs, and (4) facts indicating that the design results from a comparatively simple or cheap method of manufacturing the product. See: In re Morton-Norwich Products, Inc., supra. The record in the present case contains evidence bearing on all four of these evidentiary factors. This evidence is summarized below.
Utility Patent
Applicant has submitted a copy of a utility patent, namely, U.S. Patent No. 3,556,080 (hereinafter referred to as "the '080 patent"), issued January 19, 1971 and expired January 19, 1988, which is relevant to this case. [FN2] The invention covered by the patent is a skin allergy testing device. The drawings in the patent depict a preferred embodiment of the invention. Shown below are two of these drawings, namely, Figure 1 (the device being applied to the forearm of a patient) and Figure 4 (an end elevational view of the device):
*2 Pertinent portions of the text of the patent are quoted below:
The object of this invention is to provide an improved device for effecting multiple skin tests simultaneously by pressure punctures, and wherein the physician has full view of each scarifier being used and is able to effect substantially identical pressure punctures with each test head of the instrument, even on a nonplanar body portion, thereby insuring uniformity of testing.
A further object of this invention is to provide an improved testing instrument that is characterized by its simplicity and effectiveness in operation and by its inexpensiveness of construction.
Further objects and advantages of this invention will become apparent as the following description proceeds....
*****
In order that the spine or handle 12 be rigid and easily grasped, it is formed with substantial height, about nine-sixteenth inch relative to thickness, about one-eighth inch. The length of bar 12 is about 4 inches. This shape for handle 12 permits easy grasping by the fingers, as seen in FIG. 1, and avoids blocking of view of the various heads 16. At the same time, the handle 12 provides substantial strength in its plane, which is used to effect transfer of vertically applied forces from handle 12 through arms 14 to all the multiple-pointed heads 16 so as to effect a plurality of multiple-point pressure puncture tests simultaneously on spaced portions of the skin of a nonplanar body part, as shown in FIG. 1.
*****
While the base 18 of pressure puncture head 16 is round, the distal end of arm 14 connects to head 16 adjacent an edge of head 16 closest to spine 12. A triangular brace web 14d, lying in the plane of arm 14, is provided diametrically of the back side of base 18 and between base 18 and arm portion 14c, so as to rigidify the attachment of the multiple-pointed head 16 to the distal end of arm 14 without substantially increasing the amount of material used or obstructing the view of each head 16 and its points 22.
Each elongated arm 14 is so small in cross section as to provide relative flexibility both at the juncture of arm portion 14a with relatively rigid spine 12, and also at the elbow 14b. Thus, there may be some bending of arm 14 at its connection with handle 12 in a plurality of directions, such as both in the plane of arm 14 and transversely to said plane.
The entire instrument is integrally molded from a plastic material making the same inexpensive but effective for its intended purpose. After a single use, the instrument may be discarded, thus avoiding any infection,....
It will be understood that in applying the instrument as in FIG. 1, the physician or nurse is able to easily see which heads 16 have had their points 22 forced into the skin in a manner so as to properly elicit a test reaction. Where the body portion is not planar, only a slight rocking motion of the handle 12 is necessary to bring each head 16 into proper operative skin-puncturing association with the selected skin area. During such rocking motion, the multiple contacts of a plurality of other heads 16 with the body prevents any slippage of the instrument and thus avoids undesired scratching with points 22.
*3 While there has been shown and described a particular embodiment of this invention, it will be obvious to those skilled in the art that various changes and modifications may be made therein without departing from the invention and, therefore, it is intended in the appended claims to cover all such changes and modifications as fall within the true spirit and scope of the invention.
I claim:
1. A device for effecting multiple skin tests simultaneously on spaced portions of a nonplanar body part comprising, in combination: a relatively rigid, elongated handle, a plurality of multiple-point, skin-puncturing heads located laterally outwardly of both longitudinal sides of the handle, so that the handle does not obstruct view of the puncturing heads when being used to effect skin tests; and a plurality of elongated connector means for connecting the skin-puncturing heads to the handle; each connector means being of small size dimension relative to its length and being spaced from adjacent connector means so as not to obstruct view of the puncturing heads, each connector means extending longitudinally transversely to the longitudinal axis of the handle and interconnecting at its distal end to one of the multiple-point heads so that the heads may all be pressured from the single handle to effect a plurality of multiple-point pressure puncture tests simultaneously on spaced portions of the skin of a nonplanar body part, the connector means extending outwardly and angling downwardly of the handle means so as to locate the multiple-point heads in a plane spaced substantially below the handle means.
2. A device as in claim 1 wherein each elongated connector means provides a mounting for the multiple-point skin-puncturing head that permits flexing of each connector means in a plurality of directions at the point of connection of the connector means to the handle.
3. A device as in claim 2 wherein each elongated connector means includes an elbow section intermediate the ends of the connector means to permit additional flexibility.
4. A device as in claim 1 wherein the handle, multiple-point heads and connector means are integrally molded.
5. A device as in claim 1 wherein each elongated connector means includes brace means in the plane of the connector means and at the point of connection to a multiple-pointed head so as to rigidify the connection of the head to the connector means.
6. A device as in claim 1 wherein the handle is an elongated barlike body having a greater height than width so as to have strength for transmitting vertically applied forces from the handle to all the multiple-point heads, while affording maximum visibility of the multiple-point heads during a test-applying operation and affording easy gripping of the handle by the fingers.
7. A device as in claim 6 wherein the connector means connect to the handle adjacent the lower edge thereof, and the multiple-point heads are located in a plane spaced below said lower edge of the handle.
*4 Applicant has also submitted copies of a number of other patents, but these patents all relate to vaccination devices having only one (multiple-point) skin-puncturing "head," not multiple heads. If used in skin testing, these devices could be used to perform only one skin test at a time, rather than the simultaneous multiple skin tests which may be performed with applicant's device. Thus, the devices shown in these patents are not functional equivalents of applicant's device, but the patents do highlight the functional advantages provided by applicant's device as compared to the devices shown in the other patents.
Applicant's Advertising Materials
Applicant has submitted five pieces of advertising (i.e., three advertisements which assertedly have been run in a number of trade journals; a pamphlet; and a brochure) which feature the product configuration for which applicant seeks registration. [FN4] Most of these advertising pieces prominently feature a picture of applicant's product (referred to hereafter as "applicant's applicator") embodying the configuration sought to be registered. In addition, four of the pieces contain advertising claims relating to applicant's applicator. The following claims, each of which appears in one or more of the pieces, are representative:
8 skin tests in the time it takes to do one.
Fast, convenient procedure; simultaneously applies extracts and controls at 8 evenly-spaced test sites.
Standardizes skin test application from site to site and user to user. Deposits controlled amount of allergen at each site for highly reproducible results.
Easy to read; test and control results are reliable because they are both applied at the same time and in the same manner.
Sterile, disposable applicators have 8 sets of precision molded points.
Easy to apply; eliminates needle fear.
The eight point advantage in skin testing.
Standardizes skin testing; simultaneously applies eight tests of extracts or controls at evenly spaced sites.
Deposits standard amount of test material to uniform puncture depth, with highly reproducible results.
Uses substantially less allergen than conventional scratch/prick techniques; ....
Quick, convenient. Sterile disposable applicators can be pre-loaded for meaningful savings in set-up time.
... adequate, equal spacing between individual test sites for easy-to-read, highly reproducible results.
Availability of Alternative Designs
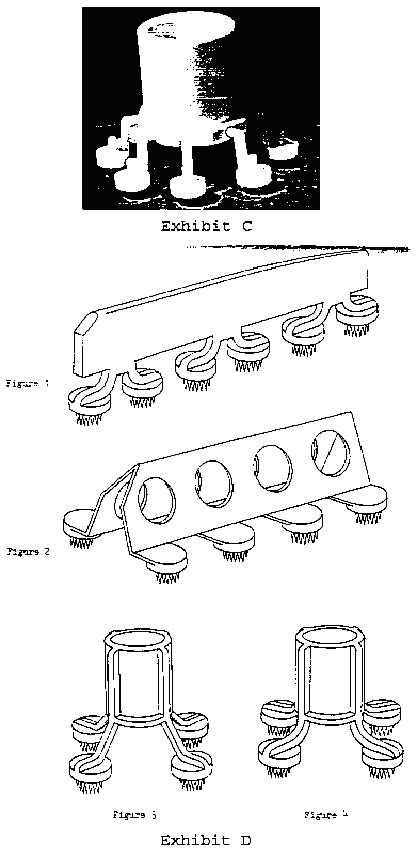
In response to the Examining Attorney's inquiry for information concerning the availability of alternative designs, applicant stated that it was not aware of any competitor which presently offers in the United States an apparatus which effects simultaneous multiple (rather than single) skin tests for detecting immediate hypersensitivity to allergens, but that alternative shapes to applicant's configuration could easily be designed. In support of the latter statement, applicant designed and submitted, with the second declaration of Gary L. Hein, seven suggested alternative shapes which, in applicant's opinion, are functionally equivalent to applicant's applicator. [FN5] Those seven alternative designs, identified as Exhibits A through D (Exhibit D depicts four designs, namely, Figures 1 through 4) to the second Hein declaration, are shown below (in reduced size):
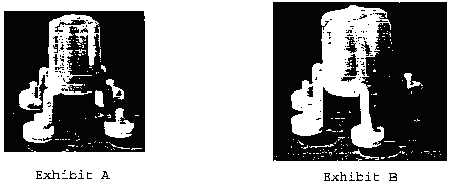
*5 In his second declaration, Mr. Hein asserts that he has over twenty years of personal experience with allergy-testing devices. Based upon that experience, he asserts, with respect to the seven suggested alternative designs, that in his opinion, each of the alternative designs differs markedly in appearance from the configuration for which applicant seeks registration; that the alternative designs will perform as well as applicant's subject design with no sacrifice of any functional advantage; that the designs shown in Exhibits A, B, and C are similar insofar as they all employ the same arm configuration; that Exhibit D shows four different alternative designs, two of which incorporate a handle having a longitudinal axis, and two of which incorporate a circular handle; that each of these four employs an arm configuration which differs from the arm configuration in Exhibits A, B, and C (and from the arm configuration of applicant's applicator); that the leg configurations of Figures 1 and 4 of Exhibit D are generally arcuate and, as such, are distinguishable from the relatively straight and downwardly and outwardly oriented legs of Figure 3 as well as the laterally extending support arms of Figure 2; and that the inverted V-shaped handle of Figure 2, Exhibit D (a modified version of the alternative design submitted as Exhibit I to the first Hein affidavit--see, in this regard, footnote 5), is as easy to grasp as the handle of applicant's applicator, will not obscure vision of the skin puncture heads, and will allow the desired degree of flexibility through control of plastic thickness, so that its arms are a full functional counterpart to the legs of applicant's applicator. Mr. Hein also asserted in the declaration that his more than twenty years of experience with allergy-testing devices has involved direct participation in the design and manufacture of loading holders for these devices; that based on this experience, he can state that loading holders for the Figure 2 "A-frame" configuration as well as for all of the other alternative configurations can be easily manufactured (for example, by thermoforming styrene) at a cost equal to or less than the cost of the loading holders used for applicant's applicator; and that once purchased by an end user, the loading holders are employed for many years.
In his third declaration, filed November 25, 1992, Mr. Hein asserted that the seven suggested alternative designs, applicant's applicator, and the design shown in the '080 patent are equally suitable for simultaneously effecting multiple skin tests to detect immediate hypersensitivity to allergens; that the alternative designs will perform as well as applicant's applicator and the patented device with no sacrifice of any functional advantage; and that the functional advantages identified in the patent are obtainable with the seven alternative designs with no sacrifice of any advantage referred to therein. In particular, with respect to the advantages identified in the '080 patent, Mr. Hein stated that each of the alternative designs:
*6 (1) Is "a skin-testing device for effecting multiple skin tests simultaneously." (column 1, lines 4-5) [FN6]
(2) Embodies "a plurality of multiple-point scarifiers in an improved device for effecting multiple spaced skin tests simultaneously." (column 1, lines 19-21)
(3) Provides "an improved device for effecting multiple skin tests simultaneously by pressure punctures ... wherein the physician has full view of each scarifier being used and is able to effect substantially identical pressure punctures with each test head of the instrument, even on a nonplanar body portion, thereby insuring uniformity of testing." (column 1, lines 33- 39)
(4) Provides "an improved testing instrument that is characterized by its simplicity and effectiveness in operation and by its inexpensiveness of construction." (column 1, lines 40-43)
(5) Includes a handle that is "rigid and easily grasped." (column 2, lines 14-15)
(6) Has a handle that "provides substantial strength ... which is used to effect transfer of vertically applied forces from [the] handle ... through [the] arms ... to all the multiple-pointed heads ... so as to effect a plurality of multiple-point pressure puncture tests simultaneously on spaced portions of the skin of a nonplanar body part, as shown in FIG. 1 [of the patent]." (column 2, lines 20-25)
(7) Includes elongated arms that "provide relative flexibility both at the juncture point of the arm portion ... with [the] relatively rigid spine ... and also at the elbow [14b see FIG. 4 of the patent]. Thus, there may be some bending of the arm ... at its juncture with the handle ... in a plurality of directions, such as both in the plane of the arm ... and transversely of said plane." (column 2, lines 42-47)
(8) Is an entire instrument "integrally molded from a plastic material making the same inexpensive but effective for its intended purpose. After a single use, the instrument may be discarded thus avoiding any infection...." (column 2, lines 48-52)
(9) Is so designed that when it is applied, "the physician or nurse is able to easily see which heads ... have their points ... forced into the skin in a manner so as to properly elicit a test reaction." (column 3, lines 23-26)
(10) Is so designed that if applied to an area "where the body portion is not planar, only a slight rocking motion of the handle ... is necessary to bring each head ... into proper operative skin-puncturing association with the selected skin area. During such rocking motion, the multiple contacts of a plurality of other heads ... with the body prevents any slippage of the instrument and thus avoids undesired scratching with the points." (column 3, lines 26-32)
Comparative Manufacturing Cost
With respect to the factor of comparative manufacturing cost, Mr. Hein's second declaration indicates that applicant's applicator is injection molded; describes the manufacturing process in some detail; states that the alternative designs would be manufactured in exactly the same manner, utilizing the same equipment; and asserts that the alternative designs can be manufactured as easily as, and at the same or less cost than, applicant's applicator. In this regard, Mr. Hein notes that the alternative designs of Exhibits A and B show a four-headed and a six-headed device, respectively, which would require less plastic resin material, and thus could be manufactured for less, than applicant's applicator; that the eight-headed device shown in Exhibit C could be produced at a cost equal to or less than the cost of producing applicant's applicator, depending on the construction (i.e., thickness, height, etc.) of the circular handle; and that all of the designs shown in Exhibit D could be produced at a cost equal to or less than the cost of producing applicant's applicator.
Other Evidence
*7 Applicant has also made of record a copy of a consent judgment entered August 10, 1990 in applicant's favor by the United States District Court for the Northern District of Illinois, Eastern Division, in Lincoln Diagnostics, Inc. v. Farris Laboratories, Inc., Civil Action No. 90 C 3123. The pertinent paragraphs of the consent judgment are quoted below:
2. Multiple head allergy skin test devices sold by Lincoln Diagnostics, Inc. in association with the MULTI-TEST trademark [FN7] have a distinctive trade dress based upon their overall shape, configuration and appearance which has acquired secondary meaning in the allergy testing field.
3. Multiple head allergy skin test devices sold by Farris Laboratories, Inc. in association with the trademark OCTATEST have an overall shape, configuration and appearance which is confusingly similar to and an infringement of the trade dress of the MULTI-TEST multiple head allergy skin test devices.
4. Defendant Farris Laboratories, Inc., its officers, agents, servants, employees and attorneys, .. are hereby permanently restrained and enjoined from offering for sale and selling the OCTATEST devices and any other multiple head allergy skin test devices having an overall shape, configuration and appearance which is confusingly similar to that of the MULTI-TEST devices. [FN8]
Evaluation of Evidence
We must now determine, based upon the facts of this case as revealed by the evidentiary record, whether the configuration of applicant's applicator is "the best or one of a few superior designs available." See: In re Morton-Norwich Products, Inc., supra, at page 16. We hold that it is.
It is readily apparent that the '080 patent, that is, the expired utility patent under which applicant was licensed, and which is described in detail above, discloses numerous utilitarian advantages of the design depicted therein. Applicant itself, in the third Hein declaration, mentions specifically ten of the functional advantages identified in the patent. [FN9] Although applicant's design is not identical to the design of the preferred embodiment depicted in the patent, the two are substantially similar in appearance and function, [FN10] and applicant has conceded that "certain utilitarian aspects of its medical apparatus were the subject" of the '080 patent. See: Page 7 of applicant's response filed August 29, 1991. Moreover, applicant's design clearly is an embodiment of the described invention, is covered by the claims of the patent, and features all of the functional advantages disclosed in the patent. Under the circumstances, we conclude that the patent discloses the utilitarian advantages of applicant's design, particularly when applicant's design is compared to single-headed designs and to the suggested eight-headed design submitted by applicant as Exhibit I to the first Hein declaration (see footnote 5).
Some of the utilitarian advantages of applicant's applicator are also mentioned in applicant's advertising materials, although in much more general terms than in the patent. For example, there are references to "8 skin tests in the time it takes to do one"; "simultaneously applies extracts and controls at 8 evenly-spaced test sites"; and "deposits standard amount of test material to uniform puncture depth, with highly reproducible results."
*8 Applicant, however, contends that its design is not de jure functional because the advantages identified in the patent and advertising are not unique to applicant's design; that the evidence of record shows that there are seven alternative designs; that each of the advantages identified in the patent is obtainable with the alternative designs; that the alternative designs will perform as well as both applicant's design and the preferred embodiment depicted in the patent with no sacrifice of any functional advantage; that the alternative designs can be manufactured as easily as, and at the same or less cost than, applicant's design; and that under the circumstances, the patent and advertising statements are evidence that applicant's design and the alternative designs have utility, but not that they are de jure functional.
The Examining Attorney criticizes applicant's alternative design evidence on the ground, inter alia, that none of the seven suggested alternatives is commercially available. We believe that alternative design evidence of this nature has probative value where, as here, there is supporting evidence as to the comparable efficacy and manufacturing cost of the suggested alternatives. See: Textron, Inc. v. U.S. International Trade Commission, supra, and In re Keyes Fibre Co., 217 USPQ 730 (TTAB 1983). However, we note that the alternative designs shown in Exhibits A and B appear to be essentially the same design, the only significant difference between them that we can discern being that the Exhibit A design has 4 arms (and heads) while the Exhibit B design has 6. Similarly, the alternative designs shown in Figures 3 and 4 of Exhibit D are very similar to one another, the only apparent difference between them being in the angle and length of the arms. Moreover, it appears to us that the test pattern created by applicant's two parallel rows of test heads, oriented in application by the T-bar, would be easier to read, in terms of comparing extracts with controls and remembering which substance was on each test site, than the circular pattern created by some of the suggested alternative designs. In addition, only the Exhibit C design and the design shown in Figure 2 of Exhibit D have the same number of heads as, and thus can perform the same number of simultaneous tests as, applicant's applicator. Of these two alternative eight-headed designs, only the Figure 2 design creates a test pattern like that of applicant's applicator. Furthermore, to achieve, with the alternative designs which have fewer than eight heads, the same number of tests run as with applicant's eight-headed applicator would require more applicators and more time, and hence more expense.
Nevertheless, assuming arguendo that there are seven alternative designs which are functionally equivalent to applicant's design, and which can be manufactured as easily as, and at the same or less cost than, applicant's design, there are still a very limited number of designs available to competitors. We believe that what we said in In re Vico Products Manufacturing Co., Inc., 229 USPQ 364 (TTAB 1985), at page 368, as quoted below, is equally applicable in this case:
*9 That there are alternative configurations of record which may work equally as well does not negate the fact that applicant's configuration was designed functionally to accomplish the purpose of supplying air and water immediately adjacent to the mixing chamber. In re Bose Corp., 772 F.2d 866, 227 USPQ 1, 5-6 (Fed.Cir.1985). As the court noted in that case:
If the feature asserted to give a product distinctiveness is the best, or at least one, of a few superior designs for its de facto purpose, it follows that competition is hindered. Morton-Norwich does not rest on total elimination of competition in the goods. (Emphasis added.)
(Additional citations omitted.) In this regard it should be noted that, while the Morton-Norwich case deals with a container design, and while the principles in that case are equally appropriate for resolving questions of registrability of product shapes, as the Bose court recognized, the manufacturers of bottles may have an infinite variety of container shapes available to them but competitors making products such as venturi bodies do not have the "infinite variety" of shapes from which to choose in producing their competitive products. (Citations omitted.) Therefore, the availability of some alternative workable product designs does not necessarily mean that applicant's design is nonfunctional. One can envision only several different alternative configurations for the positioning of the tubular air and water pipes. A registration covering one of the few alternative configurations available would, we believe, seriously interfere with the fundamental right to compete. In other words, the availability of this particular configuration is "essential to effective competition." (Citations omitted.)
See also: In re Cabot Corp., 15 USPQ2d 1224 (TTAB 1990).
Accordingly, we conclude that the configuration sought to be registered by applicant is de jure functional. [FN11]
This brings us to the issue of acquired distinctiveness. Applicant has submitted extensive evidence in support of its claim under Section 2(f) that its configuration has acquired distinctiveness as an indication of origin for applicant's goods. This evidence, which we will mention only briefly, includes the standard Rule 2.41(b) allegation of five years' substantially exclusive and continuous use in commerce; declarations concerning the nature and extent of applicant's use and promotion of the configuration sought to be registered; [FN12] declarations from 11 very prominent allergists, each of whom states that he or she is familiar with various skin testing techniques and devices (i.e., intradermal, prick puncture, scratch, bifurcated needle, Morrow-Brown needle, Keith suture needle, hypodermic syringe needle, the DermaPIK device [a single-headed device, shown in Exhibit H to the first Hein declaration--see footnote 5], and applicant's applicator), readily recognizes the configuration of applicant's applicator, considers it to be distinctive and markedly different from the configurations of other skin testing devices, and associates it with applicant; and declarations from the individuals who, over the years, have been responsible for taking phone orders for applicant's applicator and who attest to the fact that persons ordering the applicator by phone frequently identify it in a manner other than by the trademark MULTI-TEST, typically by describing or referring to the physical characteristics of the product (i.e., "little caterpillars," "little white spiders," "eight-legged testing devices").
*10 Assuming, arguendo, that applicant's configuration is not de jure functional, we find the evidence submitted by applicant sufficient to make out a prima facie case of acquired distinctiveness. Accordingly, in the event that applicant appeals our finding of de jure functionality and we are reversed on that issue, we believe that applicant's configuration should be published for purposes of opposition.
Decision: The refusal to register on the ground that applicant's configuration is de jure functional is affirmed, but the refusal to register on the ground that the configuration has not acquired distinctiveness is reversed.
J.E. Rice
E.J. Seeherman
G.D. Hohein
Administrative Trademark Judges, Trademark Trial and Appeal Board
FN1. Application Serial No. 74/100,207, filed September 24, 1990, with claimed dates of first use and first use in commerce of July, 1982.
FN2. The inventor named in the patent is Gary L. Hein, and the assignee listed therein is Lincoln Laboratories, Inc. The record herein contains three declarations by Gary L. Hein, President of applicant. The copy of the '080 patent was submitted along with the first Hein declaration, dated August 15, 1991. Mr. Hein states therein, inter alia, that on July 15, 1982, applicant acquired the business of Lincoln Laboratories, Inc. related to its multiple skin test applicators; that on the same date, applicant entered into an agreement with Lincoln Laboratories, Inc. whereby applicant obtained a license to practice the invention of the '080 patent; and that on July 28, 1982, applicant commenced selling multiple head applicators embodying the configuration sought to be registered herein.
FN3. Each of the multiple heads of the invention includes nine points clustered closely together so as to create a capillarity effect between the points for holding liquid material in the spaces between the points. The paragraphs omitted here describe two suggested systems for "loading" test materials onto (the points of) the multiple heads.
FN4. The five advertising pieces were submitted with the first Hein declaration, with each piece being set off by a divider. The five dividers were lettered "A" through "E." However, the advertising pieces did not themselves bear any corresponding letters. When the application reached the Board for final decision, the advertisements were no longer in order. For example, the Hein declaration appeared after the "B" divider, without any advertising piece, while two of the advertising pieces appeared after another divider. With the exception of a pamphlet and a brochure, submitted as Exhibits D and E, it is not possible to determine, from the information contained in the Hein declaration, the exact order (from "A" to "E") in which the five advertising pieces were originally submitted. However, the mixing of these exhibits, though inconvenient, has done no real harm.
FN5. Applicant first responded to the Examining Attorney's inquiry by submitting (as Exhibit H to the first Hein declaration) advertising materials describing a competitor's single-headed test device, sold under the mark DermaPIK, and also by designing and submitting (as Exhibit I to the first Hein declaration) a suggested eight-headed test device which applicant described as differing markedly in appearance from applicant's configuration sought to be registered. The Examining Attorney took the position that neither of these devices was the functional equivalent of applicant's applicator, because, inter alia, the competitor's product could be used to perform only one test at a time, rather than the simultaneous multiple tests which may be performed with applicant's device, and because the suggested alternative eight-headed configuration would not afford the test administrator a clear view of all of the test heads (a clear view of the test heads is necessary for determining whether all of the heads have made contact with the skin), nor would its handle be as easy to grasp as the handle of applicant's applicator. Applicant acknowledged the correctness of the Examining Attorney's observations (except for the contention concerning the Exhibit I handle). See: Applicant's Reply Brief filed August 11, 1993, footnote 4 at page 8. However, applicant then designed and submitted the seven suggested alternative shapes shown above, asserting that these seven are functionally equivalent to applicant's applicator. Accordingly, we will give no further consideration to the alternative designs shown in Exhibits H and I to the first Hein declaration.
FN6. The column and line number citations for this and the other listed advantages are to the '080 patent.
FN7. Applicant uses the word mark MULTI-TEST in conjunction with its applicator embodying the configuration sought to be registered.
FN8. In response to comments made by the Examining Attorney concerning the issue in the civil action, applicant has submitted the declaration of John L. Alex, applicant's attorney of record in the civil action. Mr. Alex avers that the complaint in the civil action charged Farris Laboratories, Inc. with a wrongful appropriation of the design and configuration of applicant's MULTI-TEST medical device in violation of Section 43(a) of the Lanham Act, not with an infringement of applicant's word mark.
FN9. In order to avoid prolonging an already lengthy opinion, we do not here enumerate the functional advantages disclosed in the patent. However, applicant's enumeration of ten such advantages is quoted earlier in this opinion, under the heading "Availability of Alternative Designs."
FN10. Counsel for applicant contends that its design differs from the design of the preferred embodiment depicted in the patent as to number of heads, and as to the appearance of the handle and heads. It is true that the two designs differ in that the embodiment depicted in the patent has twelve heads, while applicant's applicator has eight; and the handle of applicant's applicator has indentations, and a "T-bar" at one end, not included in the applicator depicted in the patent. However, it appears to us that the differences between the two designs simply add to the utilitarian advantages of applicant's applicator.
At the oral hearing held in connection with this case, counsel for applicant indicated that applicant believes that an applicator with eight or fewer heads is better than the twelve-headed embodiment depicted in the patent (and that it is for this reason that the suggested alternatives submitted by applicant have eight or fewer heads). It seems to us, in this regard, that the eight-headed design would require less material for its manufacture, and hence be cheaper to produce, than the twelve-headed design, and that because of its smaller size, the eight-headed design would be (at least to some extent) easier to use on a nonplanar body surface than the twelve-headed design.
Similarly, the indented handle of applicant's design would be easier to grasp and would require slightly less material for its manufacture. Further, according to a pamphlet distributed by applicant's distributor, Center Laboratories, submitted as an exhibit to the first Hein declaration, the T-bar on the handle of applicant's applicator serves to orient the position of the applicator during the loading of its heads with test materials ["Place Multi-Test Applicator(s) in slot(s) on Multi-Test Tray with points upright and crosspiece of 'T' bar facing you. (Applicator will fit in slot only in correct position, with numbers on test heads matching numbers next to wells for vials)."]. According to a brochure distributed by another of applicant's distributors, Barry Laboratories, and submitted with the first Hein declaration, the T-bar also serves to orient the position of the applicator during testing ["Invert and position the completely loaded Multi-Test applicator with 'T-bar' handle oriented toward the patient's head."].
As to the asserted differences in the appearance of the heads in applicant's design and in the preferred embodiment depicted in the patent, counsel does not specify how the heads of the two designs differ in appearance. To us, they appear substantially the same, with any differences between them being insignificant when viewed in the context of the entire design (except that we cannot see any numbers on the heads of the design depicted in the patent, while the heads of applicant's design are plainly numbered, an obvious advantage, for purposes of loading test materials and reading test results, over unnumbered heads).
FN11. The consent judgment made of record by applicant is of no great persuasive value on the issues presented herein because it is not a legal determination of the merits of the case, and it is just as likely to have stemmed from the defendant's desire to avoid the costs of litigation, as from a sincere belief in the validity and distinctiveness of applicant's configuration as a mark. Cf.: In re Wella Corp., 635 F.2d 845, 196 USPQ 7 (CCPA 1977). However, the consent judgment does constitute evidence that applicant has engaged in policing activity with respect to the configuration sought to be registered herein.
FN12. These declarations show, inter alia, that applicant has sold its applicator embodying the configuration sought to be registered since July 28, 1982; that between that date and August 15, 1991, when the first Hein declaration was signed, applicant sold over 7.5 million of its applicators; that since that time, unit sales of the applicator have continued to show double-digit growth; that 35% of the practicing allergists in the United States use applicant's applicator either for all patients or for nonadult patients; that applicant has promoted its applicator through appearances at trade shows and conventions, through advertisements in trade journals directed to allergists, promotional pamphlets and brochures; and that in the advertisements and promotional literature, the configuration is prominently pictured, sometimes without the word mark MULTI-TEST.