Trademark Trial and Appeal Board
Patent and Trademark Office (P.T.O.)
*1 IN RE BIO-MEDICUS, INC.
Serial No. 74/069,761
November 16, 1993
Fred Mandir
Trademark Examining Attorney
Law Office 11
(Thomas G. Howell, Managing Attorney)
Before Rice, Simms and Hohein
Administrative Trademark Judges
Opinion by Hohein
Administrative Trademark Judge
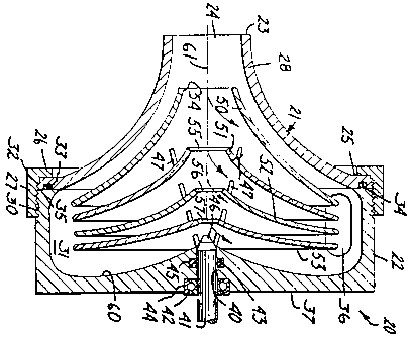
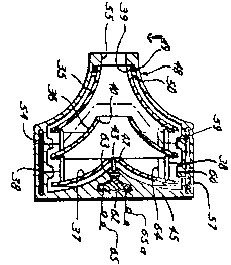
An application has been filed by Bio-Medicus, Inc. to register the configuration reproduced below as a trademark for "blood pumps". [FN1]
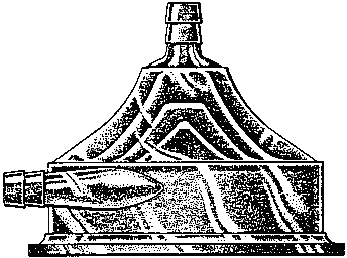
Applicant states in the application that "[t]he mark comprises a generally conical configuration for a blood pump with an internal series of concentric smooth surfaced cones visible through the outer surface". [FN2]
Registration has been finally refused under Sections 1, 2 and 45 of the Trademark Act, 15 U.S.C. §§ 1051, 1052 and 1127, on the ground that the configuration sought to be registered is de jure functional and thus is unregistrable irrespective of any claim of distinctiveness. [FN3]
Applicant has appealed. Briefs have been filed, [FN4] but an oral hearing was not requested. We affirm.
Preliminarily, we observe that as stated by the Board in In re Bose Corp., 215 USPQ 1124, 1126 (TTAB1982), aff'd, 772 F.2d 186, 227 USPQ 1 (Fed.Cir.1985), it is settled law that:
A shape or configuration of an article which is in its concept essentially or primarily utilitarian or functional cannot function as a trademark under the Federal trademark statute, and cannot be registered either on the Principal or Supplemental Register. In re Deister Concentrator Co., [Inc., 289 F.2d 496,] 129 USPQ 314 (CCPA1961); Best Lock Corp. v. Schlage Lock Co., [413 F.2d 1195,] 162 USPQ 552 (CCPA1969); In re Honeywell, Inc., 187 USPQ 576 (TTAB1975), aff'd, [532 F.2d 180,] 189 USPQ 343 (CCPA1976); In re Water Gremlin Co., [635 F.2d 841,] 208 USPQ 89 (CCPA1980); In re Lighting Systems, Inc., 212 USPQ 313 (TTAB1981); [and] In re Teledyne Industries, Inc., 212 USPQ 299 (TTAB1981). This rule applies irrespective of whether [an] applicant may have established a de facto secondary meaning in the configuration of its goods due to the existence of a patent claiming the configuration as a patentable feature or extensive advertising and promotion of the configuration over a period of time. In re Water Gremlin Co., supra, at [9]0-91. Accordingly, the threshold issue in this appeal is whether the configuration ... sought to be registered here is or is not dictated primarily by functional or utilitarian considerations.
*2 In determining such an issue, the court in the leading case of In re Morton-Norwich Products, Inc., 671 F.2d 1332, 213 USPQ 9, 15-16 (CCPA1982), outlined several general factors to be considered when evidence thereof is of record (emphasis by the court):
Keeping in mind ... that "functionality" is determined in light of "utility," which is determined in light of "superiority of design," and rests upon the foundation "essential to effective competition," ... there exist a number of factors, both positive and negative, which aid in that determination.
Previous opinions of this court have discussed what evidence is useful to demonstrate that a particular design is "superior". In In re Shenango Ceramics, Inc., 53 CCPA1268, 1273, 362 F.2d 287, 291, 150 USPQ 115, 119 (1966), the existence of an expired utility patent which disclosed the utilitarian advantage of the design sought to be registered as a trademark was evidence that it was "functional". .... It may also be significant that the originator of the design touts its utilitarian advantages through advertising. ....
Since the effect upon competition "is really the crux of the matter," it is, of course, significant that there are other alternatives available. ....
It is also significant that a particular design results from a comparatively simple or cheap method of manufacturing the article. ....
In the present case, applicant criticizes the Examining Attorney for not considering all of the Morton-Norwich factors and for "faulty" analysis of those factors which were considered. Applicant maintains that "[p]roper application of all of the Morton-Norwich factors to the evidence of record leads to a conclusion that Applicant's design is not de jure functional".
The evidence of record, all of which was furnished by applicant, consists of:
(i) The declaration of Norman McGibbon, applicant's vice president, treasurer and chief financial officer;
(ii) The declaration of Donovan Peterson, applicant's director of technical support, and the exhibits thereto, including representative advertisements showing the conical configuration used by applicant for its centrifugal blood pumps since 1975 and advertising literature for a centrifugal blood pump marketed by applicant's primary competitor, Sarns/3M;
(iii) The declaration of Allen Seck, applicant's vice president of marketing, and the exhibits thereto, including a sales brochure and a medical magazine advertisement for applicant's centrifugal blood pumps as well as articles in the "popular press" which mention and/or illustrate such goods;
(iv) The declaration of John R. Dalpee, an employee of Medtronic/Bio-Medicus, Inc. who serves as "Director of Quality Assurance/Regulatory Affairs for Applicant," and the exhibits thereto, including various utility patents pertaining to applicant's centrifugal blood pumps; sales literature and technical information concerning centrifugal blood pumps offered by Sarns/3M and another competitor, Aries Medical; a sales bulletin issued by applicant regarding prices for its centrifugal blood pumps and those offered by Sarns/3M; and further items of advertising, promotional and explanatory material dealing with applicant's current centrifugal blood pump design and earlier versions thereof; and
*3 (v) The declaration of Terry L. Wiles, a patent attorney for Medtronic, Inc., which is the owner of certain of the utility patents for applicant's goods.
The Examining Attorney maintains, on the other hand, that consideration of the above evidence in light of the Morton-Norwich factors establishes a prima facie case of de jure functionality for the configuration applicant seeks to register and that applicant has failed to rebut such case. [FN5] Inasmuch as applicant and the Examining Attorney are diametrically opposed in their views, each of the Morton-Norwich factors, and whether it supports applicant's or the Examining Attorney's conclusion, will be separately discussed.
Turning first to a review of the utility patents furnished by applicant, we note that the existence of one or more utility patents which disclose the superior utilitarian advantages of a design generally is adequate, and frequently is conclusive or incontrovertible, evidence of the de jure functionality of a configuration. See, e.g., Best Lock Corp. v. Schlage Lock Co., supra at 556 and In re Shenango Ceramics, Inc., supra. Applicant, although conceding in its substitute appeal brief that "a configuration similar to Applicant's design is illustrated as an embodiment of several utility patents owned by Applicant, namely U.S. Patent Nos. 3,864,055 (Fig. 8); 3,647,324 (Fig. 2); 3,970,408 (Fig. 1); 4,037,984 (Fig. 1); and 3,957,389 (Fig. 1)," [FN6] argues that of these patents, which constitute Exhibits 1-5 of the Dalpee declaration, "only U.S. Patent No. 3,864,055 has claims that cover blood pumps with a structure that could use a shape similar to Applicant's trademark". A representative figure is reproduced below: [FN7]
In particular, applicant stresses that as indicated in Mr. Wile's declaration, it is his opinion that while "only the claims of U.S. Patent No. 3,864,055 cover the functional structure of the configuration Applicant seeks to register as a trademark," "[t]here are, however, other blood pump configurations that cover these claims as well". Claims 7 and 8 of such patent, which are representative, [FN8] are set forth in pertinent part below (emphasis added): [FN9]
7. A method of pumping blood which is subject to damage under impact and shear, said method comprising rotating an impeller having a plurality of axially spaced smooth discs that define constant annular cross sectional vaneless pumping chambers therebetween, providing all but the axially rearmost of the discs with central openings communicating with the inner portions of said annular pumping chambers, subjecting the blood to centrifugal action by engagement with the smooth walls of the rotating discs that define the pumping chambers, increasing the outward movement of blood under laminar flow conditions without appreciable turbulance [sic] to the outer peripheries of the discs and collecting the discharged laminar flow of said blood from said pumping chambers in an annular unobstructed chamber about said discs without subjecting the blood to impact and/or shear.
*4 8. A method according to claim 7 wherein the discs converge outward of their centers.
As the Examining Attorney observed in his denial of applicant's request for reconsideration, the configuration which applicant seeks to register "clearly, realistically and proportionally displays the de jure functional features essential for any finless centrifugal blood pump". Such features include not only the input (top) and peripheral (side) outlets, but also, as stated in the description of the configuration, "an internal series of concentric smooth surfaced cones visible through the outer surface" or housing of the blood pump. [FN10] The shape and convergence of the rotators utilized in applicant's centrifugal device are in the configuration shown in order to achieve the desired degree of gentle handling and nonturbulent pumping action. This is made clear by the description of the preferred embodiments disclosed in U.S. Patent No. 3,864,055, which states in relevant part that (emphasis added):
In each of the pumps shown in FIGS. 1-6, and pumps wherein use is made of rotators (or accelerators) of the different forms shown in FIGS. 7-8, it will be noted that the rotators are designed to avoid turbulence and to avoid rapid pressuring and depressuring of the blood or other fluid being pumped, and also to avoid any physical grinding or abrasive action upon the fluid. As has been made clear, these rotator designs are made in this manner in order that blood or other delicate liquids or gases being pumped, some containing solids in suspension, will not suffer detriment and will not be destroyed by the pumping operation.
The convergence of the rotators, with diminution of the flow space there between outwardly, prevents cavitation (dissolved gases coming out of solution to form bubbles because of the pressure reduction within the pumps), which would adversely affect pumping efficiencies and cause damage to certain fluids, such as blood. ....
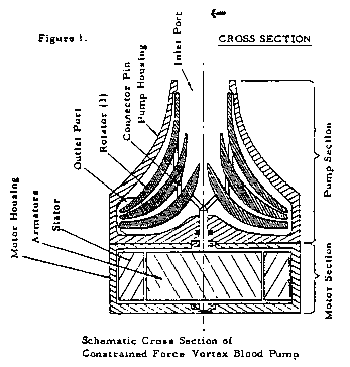
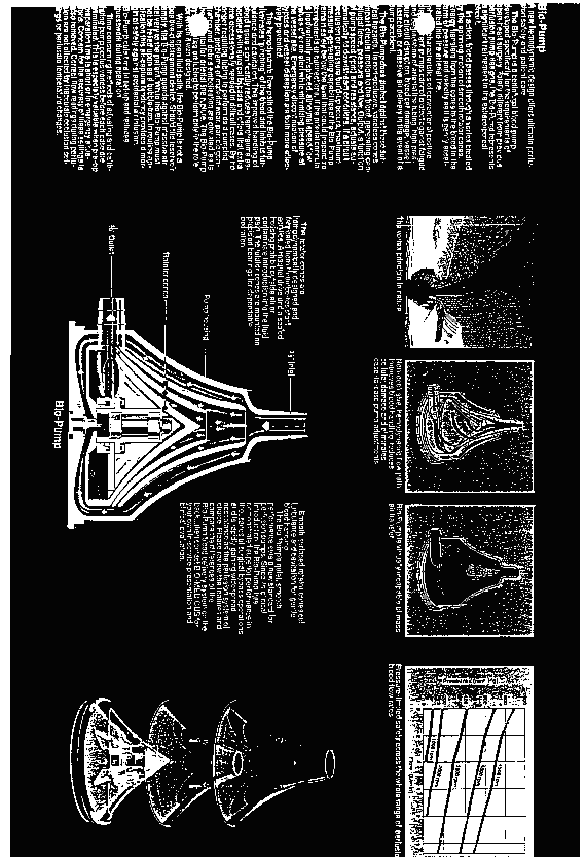
While it is also the case that applicant's utility patents disclose other rotator shapes, such as a spherical convex and a double convex rotator, which in turn would tend to dictate an exterior housing conforming to the shape of the rotator, none of the alternative designs has been demonstrated on this record to provide at least the same degree of performance as the series of concentric cones employed in the configuration for which applicant seeks registration. We especially note, in this regard, the engineering research report identified as Exhibit 28 to Mr. Dalpee's declaration. The authors of the report, which is dated March 1972 and is entitled "NONPULSATILE BLOOD PUMP," are the inventors of applicant's centrifugal blood pumps. Referring to a schematic diagram (Figure 1), reproduced in relevant part immediately below, [FN11]
which is strikingly similar to the current configuration of applicant's goods, pages 6-7 of the report discuss the essence of the blood pump under development and confirm that rotator shape, spacing and housing configuration, among other things, are crucial factors bearing upon an optimal (most efficient) design (emphasis added):
*5 The constrained force vortex blood pump consists essentially of rotating plates (rotators) within a housing as illustrated ... in Figure 1. Blood is pumped as a result of the development of centrifugal forces created by the rotary motion. This device utilizes the viscous properties of real fluids and imparts an acceleration to the blood through shear forces resulting from the relative velocity difference between the rotors of the pump and the fluid. Hence, the shear force is a function of the fluid viscosity and shear rate. After the blood enters the pump, it is pressurized and flows toward the periphery of the pump where it is discharged.
The performance characteristics of the blood pump will depend primarily upon the following design parameters or variables:
1) rotator shape (curvature)
2) number of rotators
3) spacing between rotators
4) angular velocity of rotators
6) cross-sectional area between rotators at the various radii
7) surface properties of rotators
8) housing and diffuser configuration
As a first step toward optimizing the pump, the relative importance of each of these parameters and their interdependence in determining the overall performance of the pump were assessed.
In attempting to design a pump which avoided the disadvantages of shear and turbulence inherent in impeller (finned or vaned) pumps while maintaining the high efficiency offered thereby, the inventors of applicant's goods cautioned that the optimal mechanical performance (efficiency) of a centrifugal blood pump will not necessarily be compatible with biological constraints (degree of blood trauma). [FN12] Nevertheless, based upon both qualitative data and intuitive feeling, the authors of the report arrived at the basic design shown schematically in Figure 1, which through the use of convergently spaced conically shaped rotators and other shear-reducing factors (such as coating the rotators with low-temperature isotropic carbon) minimizes the shear levels experienced by the blood's being pumped and thus results in the lowest level of turbulence of all of the designs investigated. [FN13] It consequently appears, in short, that applicant's utility patents, as well as the research report, evidence that the specific configuration presently utilized by applicant for its blood pumps takes the conical shape that it does because it not only works better than other shapes for bladeless centrifugal blood pumps, but the conical configuration utilized by applicant is the design which works best for such goods. Applicant's utility patents, when read in light of the report on the invention by its inventors, are accordingly strong if not conclusive evidence that the design chosen by applicant is de jure functional.
With respect to its advertising, applicant asserts that the promotional material it has submitted makes "no reference to any utilitarian advantages of Applicant's particular design". Although conceding that its advertisements tout the superior function of its blood pumps, applicant insists that its ads "do not claim that Applicant's design is the only design which can achieve the functional superiority". Applicant, furthermore, dismisses as mere "puffing" its advertising claims, and those of each of its competitors, that a particular blood pump is better than others. We concur with the Examining Attorney, however, that applicant touts the utilitarian advantages of the configuration of its goods and that such is consequently evidence of a superior design for blood pumps.
*6 Specifically, while the promotional brochures submitted as Exhibits 27 and 46 to Mr. Dalpee's declaration respectively praise applicant's centrifugal blood pump as the "World's Best Blood Pump" and "The best blood delivery system for bypass perfusion," the record is clear that these statements may not be discounted simply as mere puffing on applicant's part. Applicant's advertising, instead, discloses that it is the cone-shaped rotators which chiefly account for the particular advantages offered by applicant's goods. For example, Exhibit 27 states in reference to applicant's "Bio-Pump," as its conically configured product is known in the trade, that (emphasis added):
The Bio-Medicus Bio-Pump(R) operates on the Constrained Force-Vortex principle whereby smooth surfaced cone-shaped vaneless rotators do the pumping. This method of pumping is completely revolutionary, the blood in essence becoming its own prime mover. By this means of pumping there is virtually no agitation, cavitation, impact or turbulence. As a result there is negligible injury to blood elements or other constituents. A review of the world literature on blood pumps shows this method to be the gentlest method possible for circulating blood.
Exhibit 27 also makes clear that in addition to providing the lowest level of hemolysis, the design of applicant's goods provides the advantage of safer operation in that it "[e]liminates the hazards associated with tube flexion seen with conventional roller pumps". [FN14] Likewise, Exhibit 46, which is reproduced in relevant part in Appendix A, stresses the advantages possessed by applicant's goods, including the fact that it is the rotator cones which "impart circular motion to the blood" and serve to "cut turbulance [sic] and cavitation for gentle blood handling".
Similarly, the advertising brochure submitted as Exhibit 43 to Mr. Dalpee's declaration [FN15] emphasizes that applicant's "Bio-Pump(R) has neither vanes nor blades to move blood. Smooth rotator cones move blood gently with diminished turbulence and cavitation". Another of applicant's advertising brochures, furnished as Exhibit 29 to Mr. Dalpee's declaration, is to the same effect. It explains that applicant's "Bio-Pump(R) is an innovative non-occlusive blood pump developed specifically for the demands of today's more difficult open-heart surgical procedures" which works as follows: "Blood passes through a vortex created by the spinning action of smooth nested cones. Energy is then transferred from the cones to the blood in the form of pressure and velocity, gently moving the blood into the arterial line". Exhibit 38 to Mr. Dalpee's declaration, which is a further example of applicant's promotional materials, likewise notes the importance of stacked, conical rotators to "The Design Principle" employed by applicant's goods: "The BIO-PUMP operates on a centrifugal principle utilizing three cone-shaped rotators which nest together as a unit. These smooth-surfaced, vaneless rotators accelerate the blood gently, with virtually no hemolysis or other blood damage". Pictured immediately above such explanation is an illustration of applicant's conically configured blood pump. In summary, contrary to applicant's contentions, its advertising repeatedly touts the utilitarian advantages conferred by the conical shape of its goods and thus such promotion is evidence of the de jure functionality of the blood pump configuration it seeks to register.
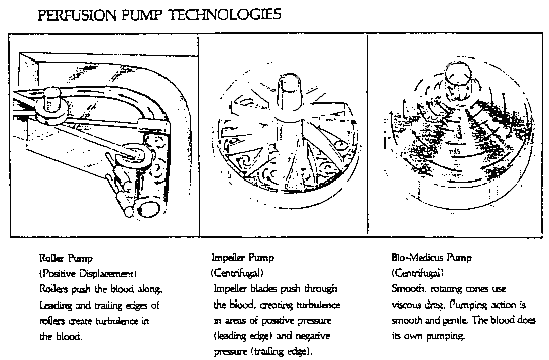
*7 Notwithstanding the foregoing, applicant insists in its substitute appeal brief that alternative designs are available for blood pumps and that competitors are in fact using such designs. [FN16] Applicant maintains that the existence of alternative designs "is 'real world' evidence" that others may successfully compete without the necessity of copying the particular configuration utilized for applicant's goods. Specifically, as set forth in an advertising brochure for its perfusion products which is designated as Exhibit 26 to Mr. Dalpee's declaration, the three basic blood pump designs presently in use are illustrated and described below:
The record indicates, however, that although all three of the technologies shown and discussed above will pump blood, in terms of patient safety and range of applications, centrifugal blood pumps are not only a major advance over roller designs, but applicant's bladeless design is a significant improvement over the impeller design employed by applicant's two competitors in their centrifugal blood pumps.
With respect to the foregoing, we especially note that as mentioned in Exhibit D to Mr. Seck's declaration, which is an article describing the successful use of applicant's blood pump which appeared in the May 7, 1984 issue of the publication Globe and Mail, "the conventional heart-lung pump, used for a few hours in open-heart surgery, ... operates by moving the blood along between rollers pushing against tubing, which damages red blood cells after an extended period. This damage also sets up a chain of reactions in the blood stream, one result of which is clotting, requiring the use of blood thinning drugs such as heparin". Another article discussing an innovative use for applicant's blood pump, which ran in the March 24, 1985 edition of The Pittsburgh Press newspaper and is attached to Mr. Seck's declaration as Exhibit G, likewise points out that traditional roller-type heart-lung machines (i.e., blood or perfusion pumps) destroy red blood cells "after about four hours" and require the use of "an anti-coagulant called heparin to keep the devices from clogging with blood clots". The article, quoting a senior heart surgeon, further states that although the use of heparin does "a very good job of keeping the pumps running, ... most of the patients were dying from massive hemorrhaging from blood that wouldn't coagulate at the surgical site".
In stark contrast to the above, the use of centrifugal blood pumps is indicated by the record to result in lower levels of blood damage than roller pumps. However, as claimed in Exhibit 27 (a 1991 advertising brochure referred to earlier), even an impeller pump is more hemolytic (destructive of blood components) than the smooth rotating cones utilized in applicant's finless goods, with the difference in the level of hemolysis becoming more pronounced the longer the pumps are in operation (up to six hours). [FN17] It is also significant that the design of applicant's goods is asserted in Exhibit 27 to do a better job at preventing the passage of blood clots inasmuch as (emphasis added): "[c]lots entering the pump tend to be trapped in the gap between cones in the Bio-Medicus pump, while impeller pumps simply pass clots toward the patient". In short, while roller pumps and impeller pumps are alternative designs available to competitors such as Sarns/3M and Aries Medical, Exhibit 27 makes clear that applicant's blood pumps, due to the superiority of their design, "have two important advantages over both roller pumps and impeller pumps:"
*8 . Bio-Medicus pumps do significantly less damage to the formed elements of blood than either roller pumps or impeller pumps.
. Bio-Medicus pumps offer a greater margin of safety to patients than roller pumps and impeller pumps in the areas of macro and micro air [bubbles], micro particles, pressure sensitivity, clots, [and] heat....
Additionally, besides the greater degree of patient safety provided by the conical design of applicant's goods, the record reflects that none of the alternative designs marketed by competitors provides the same range of applications or usefulness. Roller pumps, as well as centrifugal pumps of both the impeller and finless types, may all be used, generally speaking, in cardiopulmonary bypass surgery, although either of the centrifugal pumps provides an increased margin of safety to the patient in procedures lasting from four to six hours. However, as noted in Exhibit 43, one of applicant's advertising brochures points out that (emphasis added):
Bio-Pump is unique in its ability to perform effectively in a variety of surgical applications. The Bio-Pump has proven its superiority in over 75,000 clinical cases, resulting in ever increasing popularity with surgeons and perfusionists. They have discovered many versatile uses for the Bio-Pump which have not been matched by competitors[.]
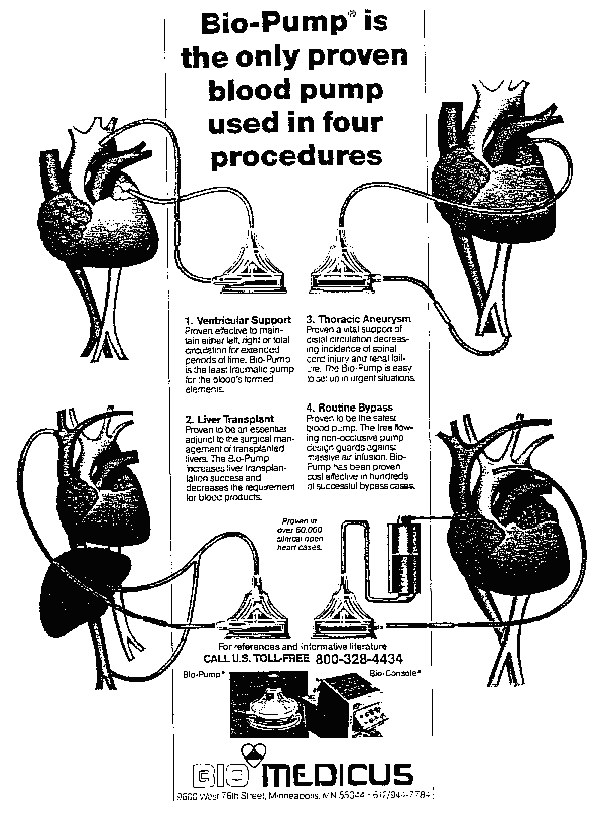
In particular, as stated in Exhibit 42 to Mr. Dalpee's declaration, which is an advertisement by applicant and is attached as Appendix B to this opinion, applicant's blood pump is used not only in routine cardiovascular bypass operations but is also utilized in cases of thoractic aneurysm, extended periods of ventricular support, and liver bypass procedures including liver transplants.
By contrast, the roller and impeller blood pumps offered by applicant's competitors are indicated for use only in cardiopulmonary bypass operations and, of even greater significance, are specifically contraindicated for use in any other kinds of longer-duration surgical procedures. For example, in Exhibit 6 to Mr. Dalpee's declaration, the "Operators Manual" for the Sarns model 7800 "Centrifugal Pump System" states on page 5 that such system, including its disposable impeller blood pump, "is indicated for use in cardiopulmonary bypass procedures" and further states, under the heading "Contraindications," that (underlining in original): [FN18]
This device is qualified only for durations appropriate to cardiopulmonary bypass procedures. It has not been qualified, through in-vitro, in-vivo, or clinical studies, for long term use in bridge to transplant or pending recovery of the natural heart.
Likewise, in Exhibit 10 to Mr. Dalpee's declaration, the instructions for use of the Aries Medical curved-vane impeller pump provide on the second page thereof that such pump "is indicated for circulatory support during cardiopulmonary bypass" and is explicitly contraindicated as follows: "Use of centrifugal pumps for periods longer than appropriate for cardiopulmonary bypass procedures may cause side effects including, but not limited to, infections, mechanical failure, hemolysis and thromboembolic phenomena". [FN19] It thus is the case that none of the alternative types of blood pumps available from competitors can be used for the broader range of applications for which applicant's conically shaped blood pumps are suitable. Competitors, in order to compete effectively in a market for more versatile blood pumps, should therefore be permitted to copy the conical configuration of applicant's products.
*9 As a final factor for consideration, applicant admits in its substitute appeal brief that it "does not possess any direct evidence which would allow it to show whether its design is cheaper to make than other competitive products". Applicant nevertheless points to statements in Mr. Dalpee's declaration as indirectly showing that applicant's blood pumps are more expensive in price than other designs and thus the configuration of its goods is not de jure functional. In particular, Mr. Dalpee avers that applicant's blood pump design competes directly with centrifugal blood pumps by Sarns/3M and Aries Medical as well as numerous roller blood pumps; that applicant's pump and the one offered by Aries Medical "are both advertised as replacements for roller pumps" and "are truly interchangeable for cardiovascular surgery"; that while applicant "has very little competitive knowledge regarding the manufacturing cost of alternative blood pumps currently on the market" since such information is not publicly available, Mr. Dalpee is of the opinion, based upon his experience and general knowledge regarding the manufacturing of blood pumps, that "the Aries [Medical] and Sarns/3M heart pumps are likely cheaper to produce than Applicant's blood pump" because such pumps "have fewer moving parts than the blood pump produced by Applicant"; and that the former, therefore, "should be cheaper to mold and assemble".
Similarly, Mr. Dalpee states that on the basis of his experience and general knowledge concerning the manufacturing of blood pumps, he is of the opinion that "the disposable portions of roller pumps are likely cheaper to produce than any of the centrifugal blood pumps currently on the market" due to the fact that "the disposable portion of the roller pump is simply a flexible piece of tubing". [FN20] Mr. Dalpee additionally notes that although he "has very little information regarding the sales price for various blood pumps currently on the market," he does know that in 1989, applicant's blood pump sold for $187 while the Sarns/3M pump sold for $159"; [FN21] and that, as of early June 1991, he does not have information with respect to the sales price for the Aries Medical blood pump. A sales bulletin distributed by applicant, which is dated September 25, 1989, accompanies Mr. Dalpee's declaration and substantiates the quoted sales prices. [FN22] The sales bulletin also indicates that applicant is willing to allow discounts for volume purchases of its blood pumps in order to compete with the nominally lower-priced pumps from Sarns/3M. [FN23]
Contrary to applicant's contention that, because its blood pumps generally sell for more than those of its competitors, its design must be more expensive to make and therefore is not de jure functional, we think that the price differential fails to prove that the configuration of applicant's goods is not functional in law. Aside from the obvious invalidity in comparing the price of a simple length of flexible PVC tubing utilized as the "disposable" portion of a roller pump with the mechanical complexity involved in a single-use centrifugal pump, we note that if purchased in larger quantities, applicant's goods are comparably priced on a unit basis with the blood pump sold by Sarns/3M. Moreover, taking at face value the pricing information furnished by Mr. Dalpee, the approximately 15% variance in the price of certain models of applicant's goods ($187) and that of the centrifugal blood pump offered by Sarns/3M ($159) would seem to be accounted for, as Mr. Dalpee points out, by the fact that the fewer moving parts of an impeller design result in a product which is "cheaper to mold and assemble" than the far more complex finless design utilized by applicant. In essence, because the principle of operation employed in a vaned pump is so different from that used in a bladeless pump, the difference in sales prices for such goods are more reflective of the advanced technology developed by applicant than a meaningful indication that the configuration of applicant's blood pumps is not functional in law.
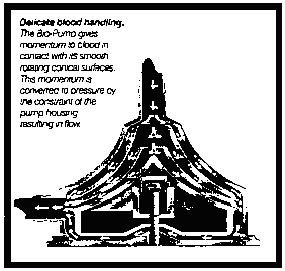
*10 In summary, as is plain from a comparison of the configuration which applicant seeks to register with the operational diagram in Exhibit 43 which is reproduced below, applicant's centrifugal blood pumps are in the conical shape shown and described in the application because such configuration was found to be the design which works best.
Analysis of the first three of the Morton-Norwich factors strongly shows that the configuration is de jure functional and the remaining factor is not persuasive of a contrary finding. Accordingly, given the expiration of U.S. Patent No. 3,864,055 and several other utility patents by applicant for its goods; the touted superiority of rotating cones within a correspondingly shaped housing as means for gently handling blood; and the lack of alternative designs which provide a useful range of operation broader than that of cardiovascular surgical procedures; we conclude that in order for others to compete effectively, they must be permitted to copy applicant's blood pump configuration. Such configuration is consequently de jure functional.
Decision: The refusal to register is affirmed.
J.E. Rice
R.L. Simms
G.D. Hohein
Administrative Trademark Judges, Trademark Trial and Appeal Board
FN1. Ser. No. 74/069, 761, filed on June 18, 1990, which alleges dates of first use of 1975. The stippling is for shading purposes only.
FN2. Pursuant to Section 2(f) of the Trademark Act, 15 U.S.C. § 1052(f), applicant also claims in the application, as originally filed, that "[t]he mark has become distinctive of Applicant's products as a result of substantially exclusive and continuous use in interstate commerce for more than five years next preceding the date of filing this application, and also as evidenced by the showing submitted separately". Applicant subsequently submitted such showing on September 10, 1990, prior to issuance of the first Office Action.
FN3. Applicant, in its substitute appeal brief, asserts that "[i]t is unclear from the final office action and the Examiner's [denial of] reconsideration whether or not the Examiner [also] determined that Applicant's mark is [inherently] distinctive or had acquired distinctiveness". However, insofar as the refusal on the ground of de jure functionality is concerned, it is pointed out that "[e]vidence of distinctiveness is of no avail to counter a de jure functionality rejection". In re R.M. Smith, Inc., 734 F.2d 1482, 222 USPQ 1, 3 (Fed.Cir.1984). Furthermore, even if the configuration which applicant seeks to register is not de jure functional, it should be noted that by including a claim of acquired distinctiveness in the application as initially filed (see footnote 2), without reserving the right to argue in the alternative, applicant has conceded that such configuration is not inherently distinctive and is not registrable in the absence of a showing of acquired distinctiveness. See In re Professional Learning Centers, Inc., 230 USPQ 70, 71 (TTAB1986). In any event, the Examining Attorney has clarified the question of acquired distinctiveness by stating in his appeal brief that "sufficient evidence was submitted by applicant to show acquired distinctiveness under Section 2(f) for a de facto functional mark, but the proposed mark is unregistrable because it is de jure functional". Thus, the issue of de jure functionality forms the only basis for refusal of registration presented by this appeal.
FN4. Although permitted by the Board to submit a supplemental appeal brief, applicant has instead furnished a substitute appeal brief together with a request that it "replace the initial brief filed by Applicant". Such request is granted. In addition, applicant has also submitted a reply brief in response to the Examining Attorney's appeal brief.
FN5. It is settled that it is incumbent upon the applicant to prove that the configuration or design of its goods is not de jure functional if a prima facie case is established that the configuration or design is functional in law. See, e.g., Textron, Inc. v. U.S. Int'l Trade Comm'n, 753 F.2d 1019, 224 USPQ 625, 629 (Fed.Cir.1985); In re R.M. Smith, Inc., supra; and In re Teledyne Industries, Inc., 696 F.2d 968, 217 USPQ 9, 11 (Fed.Cir.1982).
FN6. Applicant additionally points out that U.S. Patent Nos. 3,864,055 and 3,647,324 have expired. In particular, the 17-year period of protection provided by each patent respectively ended on February 4, 1992 and March 7, 1989. We also note that during the course of this appeal, U.S. Patent Nos. 3,957,389 and 3,970,408 expired on, respectively, May 18, 1993 and July 20, 1993.
FN7. The illustration shown is Fig. 1 of U.S. Patent No. 3,970,408, which has been turned 90<<degrees>> in this opinion (so that the fluid intake 24 is at the top of the device) to facilitate its comparison with the configuration at issue in this case. The above figure is identical to Fig. 1 in U.S. Patent Nos. 4,037,984 and 3,957,389 and, with the exception of the numbering scheme employed, is the same as Fig. 8 in U.S. Patent No. 3,864,055. Such figures are substantially similar to Fig. 2 in U.S. Patent No. 3,647,324, shown at right (in an orientation with the intake at the top), except that four nested cones are shown in the former while the latter depicts three such cones. For purposes of the patents, however, the difference is immaterial since the patent claims include references to a "plurality" of such shapes as possible configurations for the rotators of applicant's pumps.
FN8. The patent also indicates, in particular, that Fig. 8 thereof (representatively reproduced previously herein) is a "modified embodiment [] of apparatus according to the invention". Applicant, in its substitute appeal brief, points out that: "Unlike design patents, the figures attached to a utility patent do not define the claimed subject matter. They merely illustrate embodiments of the claimed invention." Nevertheless, the figures in each utility patent are part of the required disclosure of the invention.
FN9. Furthermore, by way of background, it is stated in the description of the preferred embodiments that:
Blood is a complex and delicate fluid. It is essentially made up of plasma, a pale yellow liquid containing microscopic materials and the "formed elements" which include the red corpuscles (erythrocytes), white corpuscles (leukocytes), and platelets (thrombocytes). These and the other constituents of blood, as well as the nature of suspension of these materials in blood, are fairly readily affected by the manner in which blood is physically handled or treated. Blood subjected to mechanical shear, to impact, to depressurization, or other forces, may be seriously damaged. In addition the balance between the blood constituents may readily be affected. Commencement of deterioration may result from physical mishandling of blood. Blood which has been damaged may be unfit for use.
The heart propels or pumps blood through the body in a circulating, cyclic, fashion. The blood passes repeatedly through the heart. A pump for replacing one or more pumping functions of the heart should therefore be capable of repeatedly pumping the same blood, time and time again, without damaging the blood, at least not more than to the extent where the body can function to repair or replace the blood components and eliminate damaged and waste materials therefrom.
Blood also contains dissolved and chemically combined gases, which may be seriously affected by improper physical handling of the blood. ....
....
The preferred embodiments of the invention shown and described have in common that the blood or other fluid is handled gently, without shear, shock, vibration, impact, severe pressure or temperature change, or any other condition or treatment which would unduly damage the blood or other fluid. Essentially nonturbulent flow is maintained through the pumps, and the pumped fluid is accelerated gradually and smoothly.
FN10. It is plain that the dual flarings on the outside ends of the inlet and outlet ports are functional since their purpose is to secure or hold in place the flexible tubing which is connected to such ports when the pump is in operation. It is also intuitively obvious that a conical-shaped housing requires the least area and material to enclose pumping chambers formed by nested rotator cones. Thus, if such rotator cones are a superior design which, absent patent protection therefor, others must be permitted to copy in order to compete effectively, it is manifest that applicant's configuration is functional in law and that registration is precluded.
FN11. Like the figures previously reproduced from applicant's utility patents, the figure depicted above has been oriented vertically rather than horizontally. However, except for repositioning certain captions ("Figure 1.," "CROSS SECTION" and the title of the diagram), the figure shown is unchanged.
FN12. Nunpulsatile Blood Pump Report (March 1972) at 2-4 and 8.
FN13. Id. at 44, 59 and 82. Moreover, while each prototype tested involved tradeoffs in efficiency, the inventors of applicant's goods found, for example, that using additional rotators meant a more complex design, but that such offered a lower pump speed for the same output characteristics. Id. at 82.
FN14. Specifically, while any type of centrifugal blood pump, by removing the need for a roller to occlude a length of tubing, will prevent spallation (the generation of particles created by the flexing of the tubing) and eliminate tubing rupture, disruption or splitting in the area to which a roller is repeatedly applied, the conical shape of the rotators in applicant's design offers the further advantage of precluding the sudden passage of large amounts of air through the pump and into a patient.
FN15. Such brochure is also attached as Exhibit A to Mr. Peterson's declaration and as Exhibit B to Mr. Seck's declaration.
FN16. While applicant, in its reply brief, additionally points to the several variations in rotator shape disclosed in its expired utility patents as further evidence of availability of alternative designs to competitors, we observe that, as discussed previously, none of these theoretical possibilities has been shown on the record in this case to be as optimal a configuration as the conically shaped rotators actually used by applicant. Finless centrifugal blood pumps with different rotator shapes have thus not been shown to be viable alternatives since, although such shapes will apparently pump blood, there is nothing to confirm that they do so at least as well as either a rotator of conical configuration or a nested plurality of rotators in such shape.
FN17. Applicant makes much of the fact that sellers of vaned centrifugal blood pumps tout their goods as being more efficient than the bladeless pumps sold by applicant. In particular, applicant observes that Exhibit 8 to Mr. Dalpee's declaration, which is a brochure on the Sarns/3M "Delphin" centrifugal [blood pump] system," claims greater efficiency in that "[e]fficient fins on the disposable pumphead achieve comparable flow rates with 25% fewer RPMs than finless centrifugal pumps". Such brochure further states that "[f]ewer RPMs generate less heat". In a similar vein, Exhibit 9 to the Dalpee declaration, which consists of a brochure and various advertisements for the Aries Medical "Centrifugal [Blood] Pump System," asserts that a "Curved-Vane Design Improves Flow Dynamics and Efficiency" since "[r]equired operating speed is reduced by 700 rpm across a full range of control over finless shear pumps," resulting in "[l]ess energy and heat [being] generated that could potentially damage blood elements". It is maintained by applicant in Exhibit 27, however, that contrary to the foregoing, "[a] laboratory study using infra-red measurements showed that the impeller pump generated twice as much heat as the Bio-Medicus pump" and a graph shows that at a speed of 3000 RPM, an impeller pump is more efficient only for flow rates of one liter/minute or less. Consequently, except for its greater efficiency at lower pumping rates, an impeller pump appears to offer no advantages over applicant's conical design nor, as will be noted later in this opinion, is such a pump as advantageous as applicant's pump in terms of the variety and duration of surgical procedures in which it can be used.
FN18. Among the safety warnings listed therein, the manual cautions that: "Use of this pump for periods longer than durations appropriate to cardiopulmonary bypass procedures may result in pump failure, reduced pumping capacity, excessive blood trauma, degradation or corrosion of blood contact materials with possibility of particles passing through the blood circuit to the patient, leaks, and increased potential for gaseous emboli".
FN19. The instructions further note that: "These are potential side effects with all extracorporeal blood systems".
FN20. This situation is due to the fact that the roller on a roller pump does not come into actual contact with the blood being pumped; instead, the roller simply occludes the tubing carrying the blood as it is pumped.
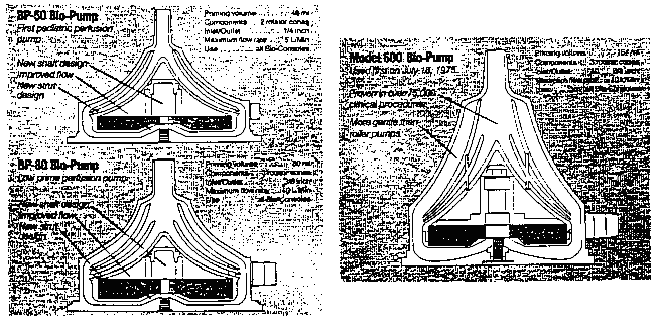
FN21. It appears from the record that the quoted price for applicant's goods refers to blood pumps bearing model numbers BP-50 and BP-80 rather than the earliest version, which applicant designates as its Model 600. However, as the following diagrams from Exhibit 43 made clear, the latest models (shown at left below) differ from the one which applicant first introduced (depicted at right below) in that the struts connecting the rotator cones have been relocated to the outer rims of the cones and the center shaft design has been refined; all of the models, however, share essentially the same conical configuration:
FN22. Such bulletin, which is designated as Exhibit 20, has a price list attached for the Sarns "7800 Centrifugal Pump System" which specifies that the prices shown therein are effective as of July 1, 1989. In particular, the price list indicates that the disposable centrifugal pump for the system is "available in cartons of 8 only," with unit prices varying as follows: $159 for 1-5 cartons; $139 for 6-35 cartons; $129 for 36-71 cartons; and $119 for 72 or more cartons.
Although the record does not contain pricing information for applicant's products as of the same date, Exhibit 30 to Mr. Dalpee's declaration, which is an advertising brochure with pricing information, discloses the following unit prices for two of the three available models of applicant's blood pumps:
---------------------------------------------------------------
Model Quantity Ordered Unit Price/pump
---------------------------------------------------------------
BP-50 12-24 Bio-Pumps (1-2 cases) $187 each
and 36-48 Bio-Pumps (3-4 cases) $175 each
BP-80 60 Bio-Pumps (5 cases) $163 each
---------------------------------------------------------------
(BP-50 and BP-80 pumps may be combined for volume discount)
Exhibit 39 of the Dalpee declaration, which is a price list for applicant's goods effective October 1, 1985, also sets forth the following unit prices:
-------------------------------------------------------------------------------
Model Quantity ordered Unit
Price/pump
-------------------------------------------------------------------------------
BP50 12 Bio-Pumps (1 case) $185
and 24 Bio-Pumps (2 cases) $175
BP80 36 Bio-Pumps (3 cases) $165
60 Bio-Pumps (5 cases) $145
$BP-50 and BP-80 pumps may be combined for volume
discount.
-------------------------------------------------------------------------------
600 12 Bio-Pumps (1 case) $245
24 Bio-Pumps (2 cases) $225
36 Bio-Pumps (3 cases) $210
60 Bio-Pumps (5 cases) $195
96 Bio-Pumps (8 cases) $190
-------------------------------------------------------------------------------
FN23. Specifically, such bulletin states that: "The Sarns' 7850 Centrifugal Pump lists for $159 each. If one was to take $30 off of the Bio-Pump list price ($187 - 30 = $157) the customer could buy two cases of Bio-Pumps with the difference they save when they buy our equipment". The bulletin concludes with the statement that: "We hope this information will give you an edge over Sarns when faced with cost objections from the customer".
Appendix A